

instituto de biologia molecular e celular | institute for molecular and cell biology
Previous Research Results:
We are interested in combining approaches of physics, chemistry and biology to understand, characterize and manipulate biological systems with molecular precision. The main lines of research are:
a) supramolecules - structural characterization and manipulation of peptide and protein assemblies. Projects: peptide-based assemblies with functional properties for molecular recognition and delivery of guest molecules; protein self-assembly processes involved in amyloid diseases.
b) molecules - protein structure elucidation using X-ray crystallography complemented with other techniques. Projects: structural and functional characterization of the enzyme responsible for the degradation of the pesticide molinate and structure-based design of transthyretin amyloid inhibitors.
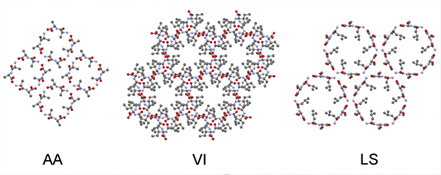
IMAGE: Peptides assemble into different lattices with different porosities. We use them to develop drug delivery vehicles and also to pursue mechanistic studies on amyloid fibril formation.
Future Research Goals:
In the future we will focus on the study of the self-assembly of biomolecules (peptides or proteins) oriented for two complementary goals: i) development of supramolecular complexes for molecular recognition and delivery of bioactive compounds and ii) mechanistic studies of amyloid fibril formation. In addition, we are also interested in using X-ray crystallography for structure-oriented research of amyloid inhibitors.
Selected References:
Rui Afonso, Adélio Mendes, Luís Gales. Peptide-based solids: porosity and zeolitic behavior. Journal of Materials Chemistry, (2011) DOI: 10.1039/c1jm13568f
Rui Afonso, Joana Durão, Adélio Mendes, Ana Damas, Luís Gales. Dipeptide crystals as excellent permselective materials: sequential exclusion of argon, nitrogen and oxygen, Angew Chemical International Edition 49 (2010) 3034-3036. Highlighted in Nature Chemistry 2 (2010) 426-427
L. Gales, L. Cortes, C. Almeida, C.V. Melo, M.C. Costa, P. Maciel, D.T.Clarke, A. M. Damas, S. Macedo-Ribeiro. Towards a structural understanding of the fibrillization pathway in Machado-Joseph´s disease: Trapping early oligomers of non-expanded ataxin-3, Journal of Molecular Biology, 353 (2005) 642-654
L. Gales, I. Cardoso, B. Fayard, A. Quintanilha, M.J. Saraiva, A.M. Damas. X-ray absorption spectroscopy reveals a substantial increase of sulfur oxidation in transthyretin (TTR) upon fibrillization, Journal of Biological Chemistry, 278 (2003) pp. 11654-11660.
Márcia Duarte, Frederico Ferreira-da-Silva, Heinrich Lünsdorf, Howard Junca, Luís Gales, Dietmar H. Pieper, Olga C. Nunes. Molinate hydrolase from Gulosibacter molinativorax ON4T - a novel cobalt dependent amidohydrolase. Journal of Bacteriology, 193 (2011) 5810-5816.
L. Gales, S. Macedo-Ribeiro, G. Arsequell, G. Valencia, M. J. Saraiva, A. M. Damas. Human transthyretin in complex with iododiflunisal – structural features associated with a potent amyloid inhibitor, Biochemical Journal, 388 (2005) 615-621.
Home | Site Map | Contacts | Credits | Privacy & Cookies | WHISTLEBLOWER CHANNEL | Intranet | Social Networks |
