
CAPTIONS:
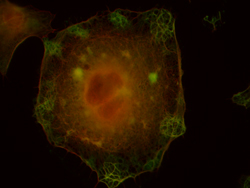 |
Kindly supplied by: Cláudia Brito, Francisco Mesquita and Sandra Sousa, Group of Molecular Microbiology, Instituto de Biologia Molecular e Celular, University of Porto, Portugal. LLO-induced membrane blebbing in HeLa cells. Green: actin-binding protein; Red: actin.
|
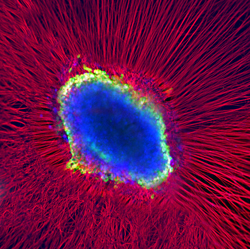 |
Kindly supplied by: Kinga Bercsenyi and Martin Wallace, Giampietro Schiavo’s Lab, UCL-Institute of Neurology, London, UK The eye of God. Whole dorsal root ganglia explant was plated into a matrigel bed and left to extend axons in all directions. We added fluorescently labelled tetanus toxin binding fragment (blue) to the culture and left it to be internalised, transported and accumulated in the cell soma of the neurons. We counterstained the culture with an antibody against beta-III tubulin (red) and DAPI (green) to reveal the axonal network and the cell bodies it originates from.
|
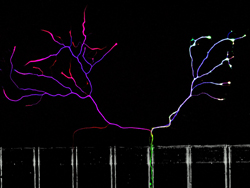 |
Kindly supplied by: Kinga Bercsenyi and Francesco Giribaldi, Giampietro Schiavo’s Lab, UCL-Institute of Neurology, London, UK The other side is always greener. Embryonic stem cell derived motor neurons (green) were plated into microfluidic chambers and left to grow their axons across the microgrooves. We added fluorescently labelled tetanus toxin binding fragment (red) to the axonal side to study its transport and subsequent transcytosis on the somal side. We counterstained the cells with an antibody against betaIII-tubulin (blue). Not only motor neurons cross in these chambers, but both these and the others cells take up tetanus toxin and transport it back to the cell body.
|
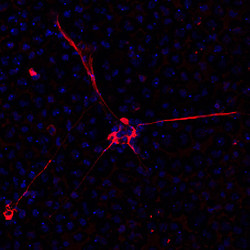 |
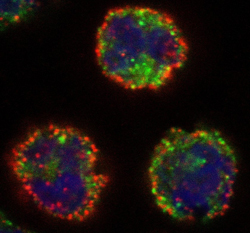
Kindly supplied by: Gilles Prévost, Institut de Bactériologie, Strasbourg, France PVL induces NETosis onto human neutrophils. Red: anti-neutrophil elastase antibody; Blue: nucleus colored with Hoechst.
|
 |
Kindly supplied by: Gilles Prévost, Institut de Bactériologie, Strasbourg, France Early binding of Alexa-labeled LukS-PV at the surface of the membrane of human neutrophils. Red: anti- LukS-PV antibody; Green: membrane labelling by B subunit of Cholera Toxin; Blue: nucleus colored with Hoechst.
|
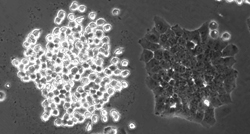 |
Kindly supplied by: Panagiotis Papatheodorou, Institute of Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, Germany Hap 1 cell clone is sensitive towards the Clostridium difficile binary toxin CDT. The right cells clone lacks the receptor for CDT and remain resistant.
|
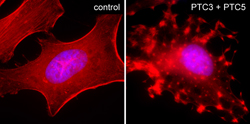 |
Kindly supplied by: Alexander E. Lang, Institute of Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, Germany Intoxication of HeLa cells with Photorhabdus luminescens ADP-ribosylating toxins PTC3 and PTC5. Cells were fixed and stained with TRITC-conjugated phalloidin and DAPI. |
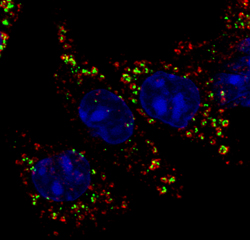 |
Kindly supplied by: Liliana M. G. Pereira, Fish Immunology and Vaccinology group, Instituto de Biologia Molecular e Celular, University of Porto, Portugal Co-localization of AIP56, an AB-type toxin from Photobacterium damselae piscicida, with the early endosome marker EEA1 in intoxicated mouse bone marrow derived macrophages. Green: AIP56; Red: EEA1; Blue: DAPI.
|
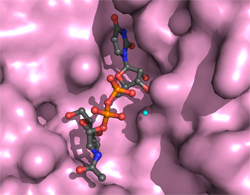 |
Kindly supplied by: Thomas Jank, Institute of Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, Germany Structure of the active site of Photorhabdus asymbiotica toxin PaTox with bound UDP-GlcNAc
|
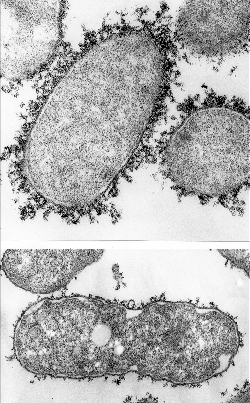 |
Kindly supplied by: Ana do Vale, Fish Immunology and Vaccinology group, Instituto de Biologia Molecular e Celular, University of Porto, Portugal Electron micrograph of Photobacterium damselae piscicida
|
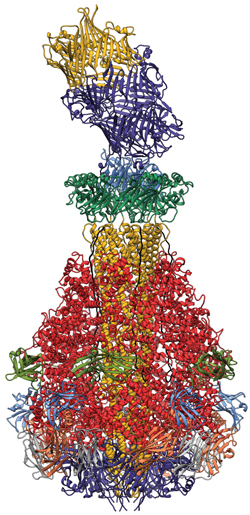 |
Kindly supplied by: Stefan Raunser, Max Planck Institute of Molecular Physiology, Dortmund, Germany Structure of the Tc toxin complex (A, B, and C component) from Photorhabdus luminescens.
|
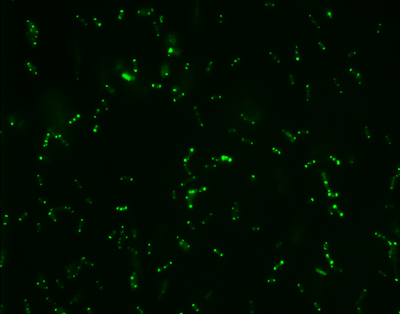 |
Kindly supplied by: Ana do Vale, Fish Immunology and Vaccinology group, Instituto de Biologia Molecular e Celular, University of Porto, Portugal Escherichia coli expressing GFP-tagged AIP56.
|
Rua do Campo Alegre, 823, 4150-180 Porto - Portugal
Tel +351 226 074 900 . Email: RITA.MATOS@ibmc.up.pt